| CATEGORII DOCUMENTE |
| Demografie | Ecologie mediu | Geologie | Hidrologie | Meteorologie |
Influenta combustibililor pe baza de acooli asupra poluarii produsa de MAS
The influence of alcohol based fuels on the pollution produced by gasoline
Rezumat:
Lucrarea de fata are ca scop prezentarea caracteristicilor de folosire a alcoolilor in locul benzinei. Prima parte a lucrarii este constituita din prezentarea teoretica a avantajelor, dezavantajelor referitoare la puterea calorica, aerul necesar arderii, componentei masice, a diferentei presiunilor de vaporizare si a altor caracteristici teoretice.
Partea a doua, partea experimentala, are ca rol punerea in evidenta a caracteristicilor de poluare comparativa la diferite turatii, folosind in prima faza benzina iar in a doua faza E30 (30% etanol, 70% benzina
Abstract
The present paperwork has as objective the presentation of characteristics of using alcohol instead of gasoline. The first part is constituted by a theoretical presentation of the advantages and disadvatages, regarding caloric power, air need in cobustion, massic component, vaporization presures and other theoretical characteristics.
The second part, experimental part, compares the polution characteristics at different ratios first using gasoline and than using E10 (10% ethanol, 900% gasoline). The experiments are made on a monocylinder engine.
1 .GENERAL FACTS
Ethanol, also called ethyl alcohol or pure alcohol is a volatile, flammable, colorless liquid.
Ethanol fuel is the same type of alcohol found in alcoholic beverages. It can be used as a fuel, mainly as a bio fuel alternative to gasoline or combined with it. Anhydrous ethanol (ethanol with less than 1% water) can be blended with gasoline in varying quantities up to pure ethanol (E100), and most modern gasoline engines will operate well with mixtures of 10% ethanol (E10).
The fact that ethanol can be easily produced using starch and sugar at a low costs combined with CO2 as only pollutant factor after combustion could allow ethanol fuels to play a much bigger role in the future than previously thought.
In the following paper it is related the research made on a mono cylinder gas engine. The main objective is to analyze the characteristics like hourly consumption, power, torque and emissions of this engine first using gasoline and than using E10 (10% ethanol, 90 % gasoline).
2. COMPARING ALCOHOLS AND GASOLINE
Caloric power
The biggest disadvantage of using ethanol is that it contains only 70 percent of the gasoline's caloric power.
Table 1. Caloric power and caloric power of mix.
|
Nr. crt |
Fuel |
Air need to burn 1 g of fuel [g] |
Caloric power of fuel [kJ/g] |
Caloric power of 1m3 of fuel mix [kJ] |
|
Methanol CH4O | ||||
|
Ethanol C2H6O | ||||
|
Gasoline |
Still, the fact that gasoline needs about 71 % more air in combustion makes the caloric power of the mixture between gasoline or ethanol and air to be almost the same.
Mass components
Alcohols contain in their molecule oxygen fact that determines the combustion to have less pollutant emissions and a higher burning speed. Because of this higher burning speed, commonly, the functional engine's parameters could be affected (the apparition of detonation at certain working conditions of load and rpm).
Table 2. Mass composition
|
Nr. crt |
Mass components % |
Methanol |
Ethanol |
Gasoline |
|
C | ||||
|
H | ||||
|
O |
3. Octane number
Ethanol's octane higher number (113) comparing to gasoline (95-98) can be seen in a increase of power by using ethanol of 10 to 20 percent.
Even if ethanol has 30 percent less energy the fuel consumption increases by only 10-20 percent
The increase of power comes from two factors: a higher octane number and a higher vaporization heat, cooling the mixture at the entrance in the engine, increasing the ratio Tmax / Tmin in the thermic cycle, making the total efficiency to increase.
4. Vaporization
Still, a lower temperature of the mix that is going to be introduced in the cylinder can conduct the mix to reach of the "dew point" that will lead to the condensation of the fuel in the admission system.
Table 3
|
Nr.crt |
Fuel |
" Dew point" [oC] |
The decrease of the temperature by vaporizing the fuel [oC] |
|
|
Ethanol C2H6O | ||
|
Methanol CH4O | |||
|
Gasoline |
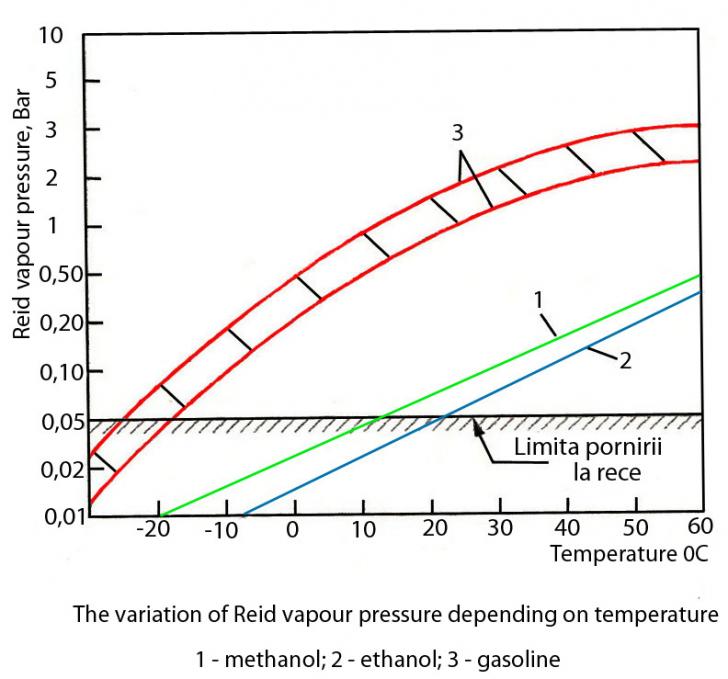
1
5. The laminar burning speed.
2
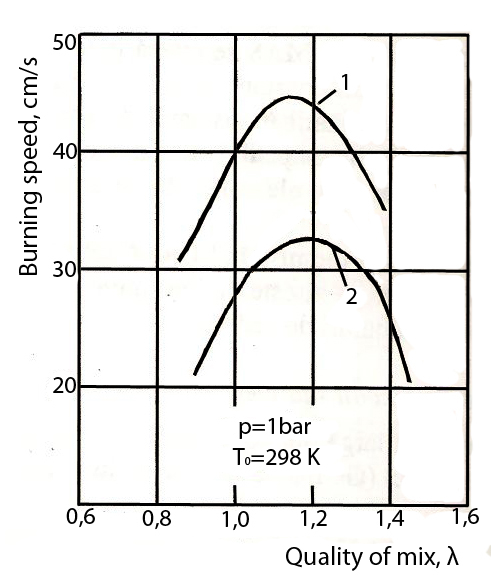
As a result, the time periods of initiating a normal burn are shorter in an engine fueled with ethanol.
Variation of the burning speed depending on the quality of the mix
Methanol
Gasoline
THE EXPERIMENT

3
I II
The
first measurement was 4
In gasoline's case, every time the engine speed was decreasing by 200 rpm, the measurements were repeated. This process was done until the engine speed was around 2400 per minute.
Those are the values that were measured for gasoline:
Table 4. Measured values for gasoline
|
Nr.crt |
n [rpm] |
Ch [kg/h] |
M [N*m] |
P [kW] |
|
CO [%vol] |
HC [ppm] |
NO [ppm] |
|
| ||||||||
Table 5. Measured values for E10
|
Nr.crt |
n [rpm] |
Ch [kg/h] |
M [N*m] |
P [kW] |
|
CO [%vol] |
HC [ppm] |
NO [ppm] |
For each parameter graphics depending in engine's speed were made using Mathcad soft.
Hourly consumption depending on engine's speed.
Variation of momentum
depending on engine's speed.
Variation of Power
depending on engine's speed.
Variation of the temperature
on the exhaust depending on the engine's speed.
on the exhaust depending on the engine's speed.
Variation of the quantity of HC
depending on the engine's speed.
Variation on the quantity of NO depending on the engine's speed.
In E10's case, the whole process had to stop when the rpm was somewhere near 2600 because detonation started to appear repeatedly. This was happening because the E10's burning speed is higher than gasoline's. This could be corrected only by modifying engine's advance to spark.
Bibliography
[1] Bataga, N. Burnete, I. Barabas, s.a Combustibili, lubrifianti si material special pentru automobile. Economicitate si poluare. Editura Alma Mater, Cluj Napoca, 2003, ISBN 973-8397-37-5.
[2] Eduard Rankosi, Radu Rosca
s.a, Combustibili neconventionali oxigenati pentru motoare cu ardere interna.
Editura Gheorghe Asachi,
|
Politica de confidentialitate | Termeni si conditii de utilizare |

Vizualizari: 2450
Importanta: ![]()
Termeni si conditii de utilizare | Contact
© SCRIGROUP 2025 . All rights reserved